Authorization Procedures
There are several different authorization procedures that a company may use to obtain a marketing authorization for a medicinal product depending on the consent of the company.
- National Procedures
- Harmonization and Collaborative Procedures
National Procedure
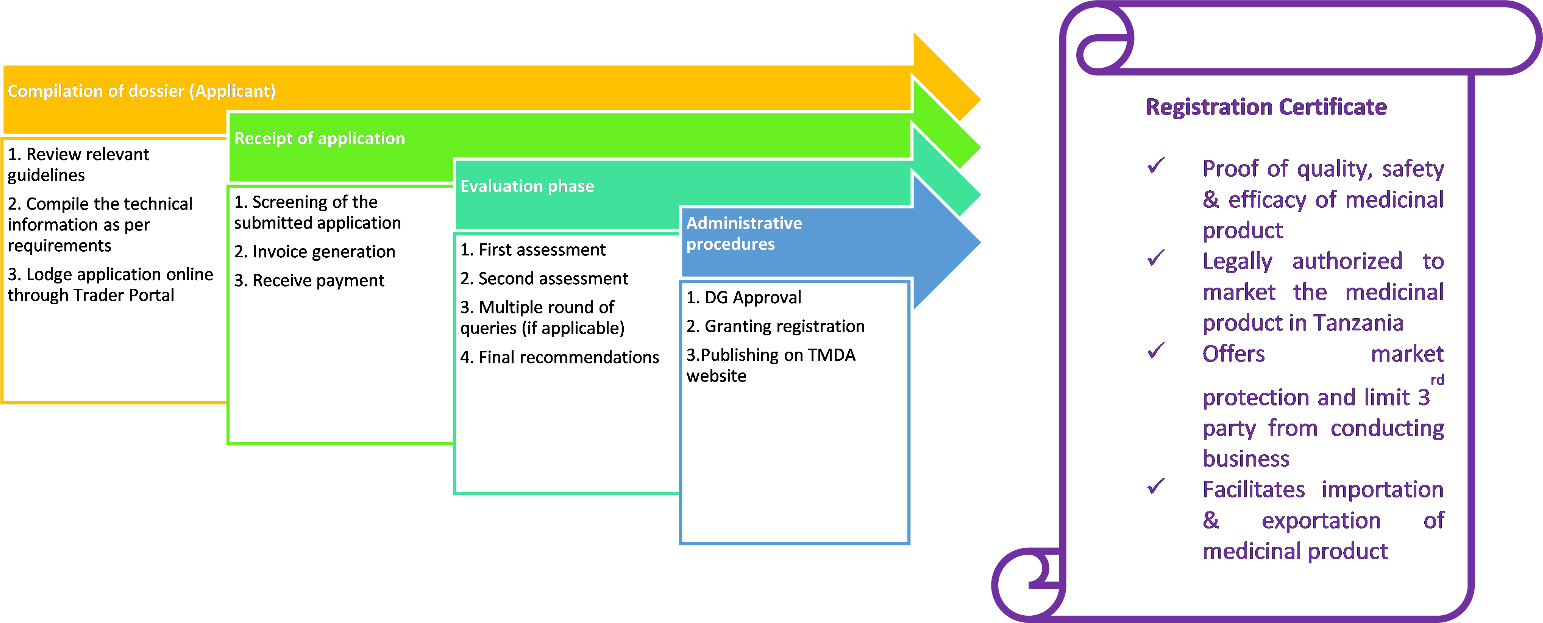
A company can submit an application for a marketing authorization directly to the TMDA if the company only wishes to market a medicine in Tanzania. The procedure for marketing authorization of medicinal product in Tanzania are described below: -
New Products
Authorizations to market new medicinal products are granted by the TMDA under the Tanzania Medicines and Medical Devices (Registration of Medicinal Products) Regulations, 2015. Authorization for a medicinal product is called marketing authorization. Details on the content of an application are outlined in the Registration of Medicinal Products Regulations and in product specific guidelines all of which are available on the The TMDA website (link to the guidelines). All applications have to be submitted in accordance with the CTD (Common Technical Document) format. Details on the format of the dossier are outlined in respective product guidelines
Process flow
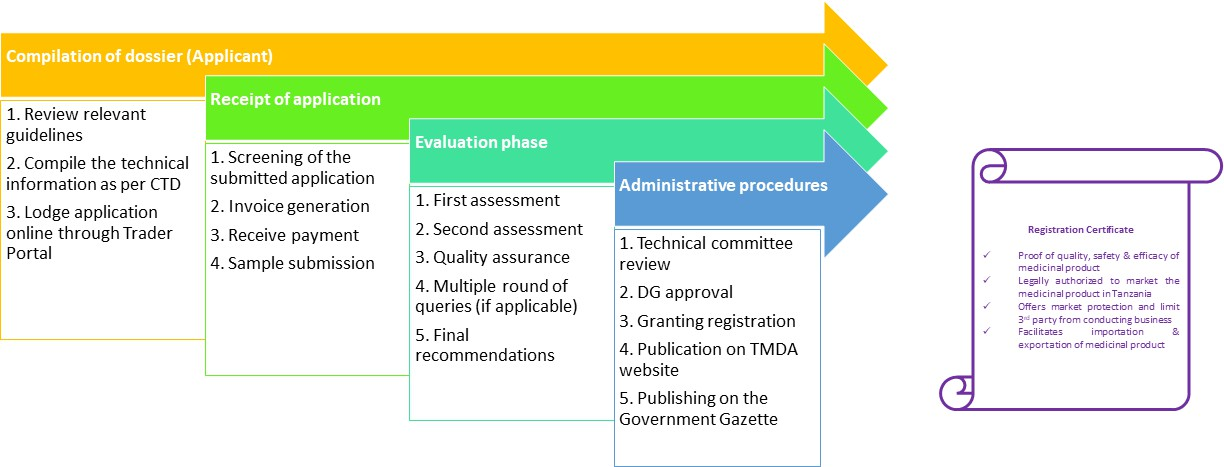
Authorization of Variations
Information for applicants on the process and requirements for notification and approval of changes to the terms of a marketing authorization.
After a medicine has been authorised, the terms of the marketing authorization may subsequently be varied. The procedures around such variations are governed by Tanzania Medicines and Medical Devices (Registration of Medicinal Products) Regulations and in product specific variation guidelines all of which are available on the The TMDA website. Tanzania Medicines and Medical Devices (Registration of Medicinal Products) Regulations, 2015 extends the scope of the variation’s regulation to all marketing authorizations, human, herbal and veterinary, granted marketing authorization. Detailed procedural guidance on the classification, submission and processing of variations has been published by the product specific variation guideline
Process flow
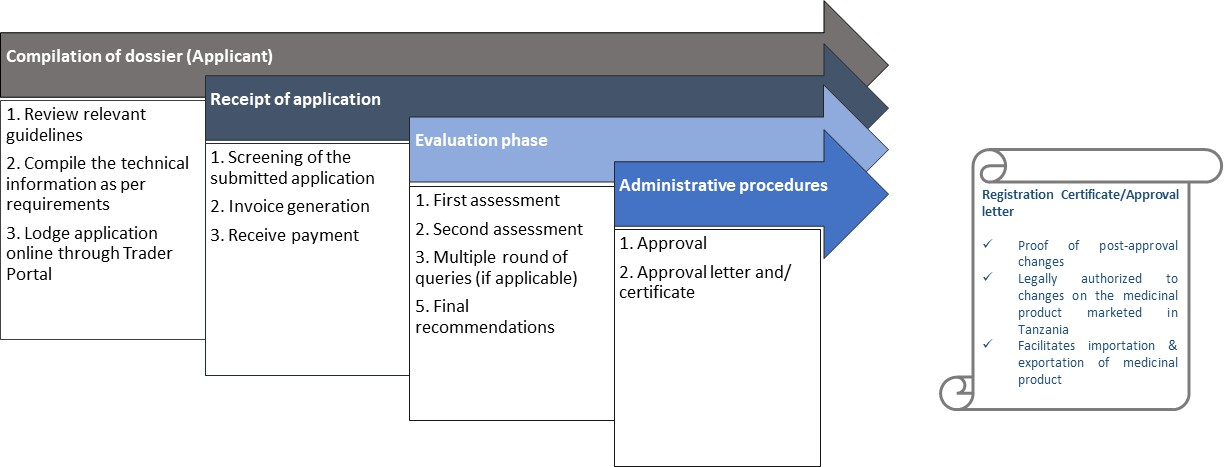
Renewal applications
Marketing authorizations are valid for five years from the date of first issue. For the authorization to remain valid, it should be renewed at the end of this five year period.
Renewal applications should be submitted to the TMDA at least three (3) months before the expiry of the authorization, although earlier renewals are acceptable in order to facilitate a common renewal date for a range of products. Renewal applications should be accompanied by the Application form for renewal of a marketing authorization.
For more information on preparing renewal applications, please refer product specific guidelines all of which are available on the The TMDA website
Process flow